- 30-week outcomes from the Part 4 INHALE-3 research develop upon the constructive 17-week information offered earlier this yr on the American Diabetes Affiliation’s 84th Scientific Periods
- Switching to, or remaining on Afrezza allowed twice as many individuals to get to objective in the course of the extension part
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Sept. 30, 2024 (GLOBE NEWSWIRE) — MannKind Company MNKD, an organization targeted on the event and commercialization of inhaled therapeutic merchandise and gadgets for sufferers with endocrine and orphan lung ailments, as we speak introduced top-level 30-week outcomes from its Part 4 INHALE-3 research, wherein further sufferers residing with kind 1 diabetes achieved goal A1c ranges in the course of the extension part. The completer evaluation which included all folks on inhaled insulin, evaluated two separate teams – one which utilized Afrezza® (plus basal insulin) over 30 weeks, and a second group of sufferers who switched to Afrezza at week 17 from common care, outlined as a number of each day injections (MDI), an automatic insulin supply system, (AID) or a pump with out automation.
“The information from the extension part of this research confirmed that extra folks residing with T1D are in a position to attain goal A1c ranges after they stay on Afrezza (plus basal insulin) or swap to Afrezza from common care – whether or not they’re utilizing a number of each day injections or pumps,” mentioned Michael Castagna, PharmD, Chief Govt Officer for MannKind Company. “We consider this information demonstrates to healthcare practitioners that Afrezza is an efficient device for his or her sufferers who need to enhance their glycemic management.”
Key Findings:
There was continued enchancment within the Afrezza (plus degludec)-treated group, with further topics reaching A1c <7% at 30 weeks – a 100% enhance from baseline:
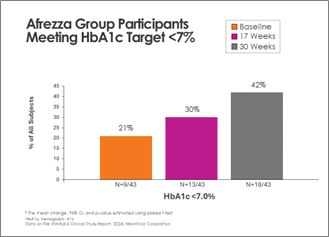
Switching from common care to Afrezza (plus degludec) at week-17 allowed greater than double the topics to attain A1c <7% at week-30, in comparison with the quantity at objective at week-17:
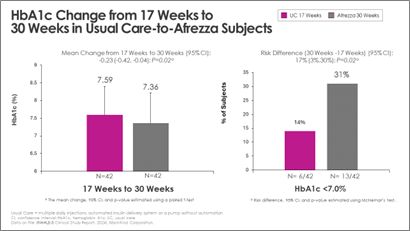
“With the constructive information acquired at each 17- and 30-weeks, we proceed to affirm that Afrezza is a crucial choice for grownup sufferers managing their diabetes,” mentioned Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Space Head, Endocrine Ailments for MannKind Company. “We sit up for discussing extra particulars of the 30-week research outcomes at ATTD subsequent March and extra conferences in 2025.”
Concerning the INHALE-3 Research
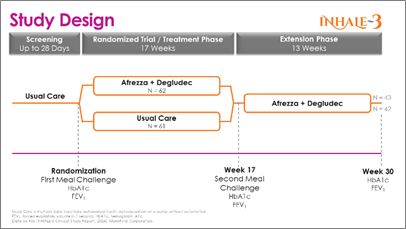
The INHALE-3 research is a 17-week, randomized managed trial with a 13-week extension performed throughout 19 U.S. websites. The research, which enrolled 141 sufferers (123 randomized), assigned individuals over 18 years of age with T1D who’re utilizing MDI, an automatic insulin supply system, or a pump with out automation to both proceed their commonplace of care or provoke an insulin routine of a each day basal injection plus Afrezza for boluses (mealtime and corrections). Topics using Afrezza (inhaled insulin) acquired a better preliminary conversion dose than within the present U.S. product label. Each arms utilized steady glucose monitoring to evaluate glucose management.
The randomized management trial (RCT) included an inhaled insulin group that started with 62 topics at randomization and 57 at 17 weeks; the standard care group consisted of 61subjects at randomization and 58 at 17 weeks. The 17-week results beforehand shared that the research met its main efficacy endpoint of a non-inferior change in HbA1c between baseline and week 17 in comparison with the standard care group. At 17 weeks, those that utilized Afrezza (plus basal insulin) continued with it via the extension part, and those that had been on common care converted to Afrezza to week 30. The extension part began with 45 topics from the inhaled insulin group and 43 accomplished the extension; the standard care-to-Afrezza group began with 49 within the extension, with 42 finishing. There was no management group within the extension part. A1c ranges had been obtained at baseline, 17 and 30-weeks.
Extra data on the INHALE-3 research is out there at: ClinicalTrials.gov(NCT05904743).
About Afrezza
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to enhance glycemic management in adults with diabetes mellitus.
Limitations of Use: Not beneficial for the therapy of diabetic ketoacidosis or in sufferers that smoke or have not too long ago stopped smoking.
Vital Security Info
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been noticed in Afrezza-treated sufferers with bronchial asthma and COPD
- Afrezza is contraindicated in sufferers with persistent lung illness resembling bronchial asthma or COPD
- Earlier than initiating Afrezza, carry out an in depth medical historical past, bodily examination, and spirometry (FEV1) to determine potential lung illness in all sufferers.
Commonest opposed reactions are hypoglycemia, cough, and throat ache or irritation.
Please see further Vital Security Info, Full Prescribing Info, together with BOXED WARNING, out there on Afrezza.com/security.
About MannKind
MannKind Company MNKD focuses on the event and commercialization of modern inhaled therapeutic merchandise and gadgets to deal with severe unmet medical wants for these residing with endocrine and orphan lung ailments.
We’re dedicated to utilizing our formulation capabilities and system engineering prowess to reduce the burden of ailments resembling diabetes, nontuberculous mycobacterial (NTM) lung illness, pulmonary fibrosis, and pulmonary hypertension. Our signature applied sciences – dry-powder formulations and inhalation gadgets – provide speedy and handy supply of medicines to the deep lung the place they will exert an impact domestically or enter the systemic circulation, relying on the goal indication.
With a passionate crew of Mannitarians collaborating nationwide, we’re on a mission to provide folks management of their well being and the liberty to dwell life.
Please go to mannkindcorp.com to study extra, and comply with us on LinkedIn, Facebook, X or Instagram.
Ahead-Trying Statements [to be updated as necessary]
This press launch incorporates forward-looking statements concerning the deliberate launch of outcomes from a scientific research that entails dangers and uncertainties. Phrases resembling “believes”, “anticipates”, “plans”, “expects”, “intends”, “will”, “objective”, “potential” and related expressions are meant to determine forward-looking statements. These forward-looking statements are primarily based upon MannKind’s present expectations. Precise outcomes and the timing of occasions might differ materially from these anticipated in such forward-looking statements on account of numerous dangers and uncertainties, which embody, with out limitation, the danger that we might not obtain our projected improvement objectives within the timeframes we count on in addition to different dangers detailed in MannKind’s filings with the Securities and Change Fee, together with its Annual Report on Type 10-Ok for the yr ended December 31, 2023, and subsequent periodic reviews on Type 10-Q and present reviews on Type 8-Ok. You might be cautioned to not place undue reliance on these forward-looking statements, which communicate solely as of the date of this press launch. All forward-looking statements are certified of their entirety by this cautionary assertion, and MannKind undertakes no obligation to revise or replace any forward-looking statements to mirror occasions or circumstances after the date of this press launch.
AFREZZA and MANNKIND are registered emblems of MannKind Company.
Photographs accompanying this announcement can be found at
This press launch was revealed by a CLEAR® Verified particular person.

For MannKind: Media Relations Christie Iacangelo, (818) 292-3500 E-mail: [email protected] Investor Relations Ana Kapor E-mail: [email protected]
© 2024 Benzinga.com. Benzinga doesn’t present funding recommendation. All rights reserved.